Forschung
Seiteninhalt
- Project 1 - Engineering autologous dermo-epidermal skin grafts
- Project 2 - Maintaining the keratinocyte stem cell compartment in cell culture
- Project 3 - Identifying novel sources of human keratinocyte stem cells
- Project 4 - Optimizing the biomatrix
- Project 5 - Engineering pre-vascularized skin grafts
- Project 6 - Colour adjusted engineered autologous skin composites
- Project 7 - Clinical Phase I and Phase II trials
- Project 8 - Tracing keratinocyte lineages in bio-engineered human skin
Project 1 - Engineering autologous dermo-epidermal skin grafts
Our goal is to develop autologous dermo-epidermal skin composites (full thickness skin analogues) that can be used clinically to cover skin defects of any origin in one single surgical intervention. To achieve that we are testing different biodegradable matrices which serve as scaffolds for keratinocytes, melanocytes, endothelial cells and fibroblasts.
Project 2 - Maintaining the keratinocyte stem cell compartment in cell culture
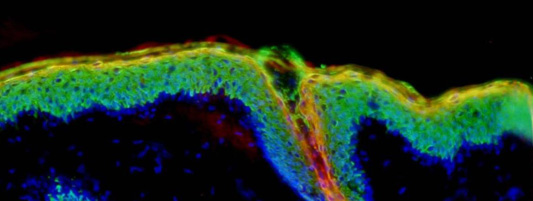
Epidermal regeneration in native and in vitro engineered skin, indispensably requires the presence of self renewing keratinocyte stem cells. To identify markers specific for keratinocyte stem cells, we screened a series of antibodies for their exclusive binding to the human hair follicle bulge (where those stem cells are known to have their niche). In a second step, these antibodies were used to identify basal keratinocytes and potential epithelial stem cells in the human epidermis and in engineered skin substitutes. (Pontiggia 2009).
Project 3 - Identifying novel sources of human keratinocyte stem cells
To determine whether human sweat glands harbour keratinocyte stem cells (in analogy to the hair follicle bulge region), we developed engineered skin grafts containing human sweat gland cells. We demonstrated, the capability of human eccrine epithelial sweat gland cells to form a stratified interfollicular epidermis substitute on collagen hydrogels both, in vitro and in vivo. From these data we conclude that in analogy to the human hair follicle bulge region, also human eccrine sweat glands harbour potential keratinocyte stem cells (Biedermann 2010).
Project 4 - Optimizing the biomatrix
The bio-engineering of skin for the generation of large transplantable dermo-epidermal skin replacements is dependent on a three-dimensional matrix that supports the biological function of skin cells and provides mechanical properties to allow for surgery. Until now, collagen type I hydrogels promise the best biological functionality but their mechanical weakness and tendency to contract and degrade do not allow for the generation of large transplants. We have proven that by plastic compression, collagen hydrogels can acquire mechanical and biological stability, while maintaining excellent biological functions. Cultured dermo-epidermal skin grafts based on compressed collagen hydrogels can be handled easily in clinically relevant sizes, do degrade at an appropriate rate, and give rise to near normal homeostatic skin (Braziulis 2012).
Project 5 - Engineering pre-vascularized skin grafts
Initial take, development, and function of transplanted engineered tissue substitutes are crucially dependent on rapid and adequate blood perfusion. Therefore, the development of rapidly and efficiently vascularized tissue grafts is vital for tissue engineering and regenerative medicine. Our goal is to develop pre-vascularized human skin grafts and to evaluate the effects of pre-vascularization on skin regeneration in vivo. We were able to generate a network of highly organotypic, branching, lumen-forming capillaries in engineered skin substitutes. After transplantation onto immuno-incompetent rats, histological analyses of the pre-vascularized skin substitutes showed that the engineered human vessels quickly connected to the vasculature of the recipient animal. This became obvious because the bio-engineered capillaries of human origin contained red blood cells of rat origin, and rapidly supplied the graft with oxygen and nutrients. (Montaño 2010 and Luginbühl 2012, manuscript in preparation).
Project 6 - Colour adjusted engineered autologous skin composites
Although current tissue engineered autologous dermo-epidermal substitutes show a multilayered epidermal structure with an excellent mechanical protective function, no structured approach to melanocytic integration has been aimed for to date. This leads not only to insufficient biological protective function against radiation but also to randomly hypo- or hyperpigmented transplanted areas on the patient. Our aim is to systematically add melanocytes to autologous substitutes to achieve an additional protective function as well as an aesthetic improvement of autologous dermo-epideramal skin substitutes
Project 7 - Clinical Phase I and Phase II trials
After more than 11 years of research the Tissue Biology Research Unit (TBRU) of the Department of Surgery of the Children’s Hospital in Zurich has come up with new skin grafts for Regenerative and Transplantation Medicine. The complex, bioengineered skin grafts resemble the properties of normal human skin as closely as possible. The close collaboration between the TBRU and the Pediatric Burn Center of the Department of Surgery of the Children’s Hospital in Zurich made it possible that the above-mentioned skin grafts can be transplanted in just one surgical intervention. The new skin grafts are intended to be applied in clinical disciplines, such as Burn and Plastic Surgery and Dermatology, in Zurich and in Europe. The development of the grafts was, and is, based on findings gained by the knowledge and methods of basic research in cell and molecular biology. The translational medical project aims at Clinical Trials that will be undertaken in 2014 by the EuroSkinGraft, EU FP7 Consortium (coordinated by the TBRU) and in close collaboration with the Pediatric Burn Center, the Clinical Trial Center (CTC) in Zurich, and the Swiss Center for Regenerative Medicine (SCRM) in Zurich.
Project 8 - Tracing keratinocyte lineages in bio-engineered human skin
Different subpopulations of epidermal keratinocytes can be identified by the expression of specific cytokeratins and other protein markers. Unfortunately, self-renewing epidermal keratinocytes do not specifically express any known stem cell marker(s). This project aims at the definition of the role of particular keratinocyte populations expressing particular markers, and, eventually, at the identification of self-renewing epidermal keratinocytes.
The project applies both conventional and more recent (molecular) tools and methods (such as CRISPR/Cas9, inducible CRE-LoxP, suicide gene depletion) for the generation of lentiviral vectors to study both the overexpression and inactivation of certain genes, and the depletion and lineage tracing of particular keratinocyte populations. Modified cells will be introduced into dermo-epidermal skin substitutes, and skin regeneration will be analysed both in vitro and in vivo.
